Related article: Supply chain and logistics modeling: how to choose the right approach.
The specific requirements of cold chain logistics in pharma became a global concern during the COVID-19 pandemic. Vaccines needed rapid worldwide distribution at an unprecedented scale. Simulation modeling played a key role in successfully meeting the challenge.
This article shows how the consultancy company Skkip created an appropriate cold chain solution and benefitted from using anyLogistix software in their work.
Contents:
A cold chain is a supply chain for goods that must be kept in specific low-temperature conditions. They are used to preserve the high quality and safety of such products as seafood, frozen food, chemicals, and pharmaceuticals.
In 2019, cold chain accounted for more than a quarter of all pharmaceutical logistics and is expected to grow further from 2021 to 2028. The need for cold chain comes from the temperature sensitivity of pharmaceuticals such as some medicines and vaccines, which can denature and become ineffective, dangerous, or even life-threatening.
Among other pharmaceuticals, the transportation of temperature-sensitive vaccines is a demanding task. These life-saving products must be moved carefully throughout the supply chain with perfectly coordinated temperature-controlled logistics activities.
Consistent temperature control is important because these vaccines must be kept within a certain temperature range from the time they are manufactured until the moment they are administered.
If the vaccine’s temperature goes above or below the required range, it can lose its ability to protect against the disease it is targeting – it can lose its potency.
Example: The two most popular COVID-19 vaccines – Pfizer-BioNTech and Moderna – require frozen and deep-frozen temperatures. They have set new standards for ultra-cold chains as they need to be maintained between -90°C and -60°C (-112°F to -76°F) and at -25°C and -15°C (-13°F and 5°F) respectively.
The main advantage of using a simulation approach for modeling a vaccine supply chain is its systemic and holistic view of reality. Such a view allows supply chain scenario testing, such as the testing of inventory management policies, and the identification of bottlenecks.
Overall, simulation modeling increases supply chain visibility end-to-end and its results support strategic decision-making and investment allocation.
Related article: Supply chain and logistics modeling: how to choose the right approach.
Vaccine production usually starts with the preparation of its components: bulk (the drug substance) and adjuvant (which promotes a stronger immune response). This stage is then followed by the formulation step where the substance and the adjuvant are combined into a vaccine.
At the filling step, containers (vials) are filled with the formulated vaccine. The product then goes to the final packaging stage, which will have specific requirements for different regions and compliance with local laws. Vials are then packaged in larger boxes and in secondary outer packaging ready for distribution.
The vaccine production process requires facilities for raw component manufacture, formulation, filling, and packaging of the finished product.
Compared with other industries, the supply chain for vaccines is relatively straightforward until production. But from the production stage through until usage, the supply chain for temperature-sensitive vaccines is a complex process because of the cold-chain logistics requirements.
Vaccine distribution planning has its difficulties:
Related article: How to manage supply chain disruption.
COVID-19 vaccine supply chains have specific constraints, as discussed, and the related high levels of uncertainty may shift conventional vaccine delivery paradigms. In this case, traditional optimization tools will not be enough to provide actionable results.
Simulation modeling of a COVID-19 vaccine supply chain provides insights into its dynamics and trade-offs. These insights help manufacturers understand the structural complexity of the overall system and predict the quantity and the timing of deliveries.
In this case study, anyLogistix supply chain design and simulation software was used to test different scenarios and determine maximum productivity under specific constraints.
Related article: Epidemic impacts on supply chain – analysis paper.
The consultancy team modeled a global supply chain with facilities located in different geographical locations:
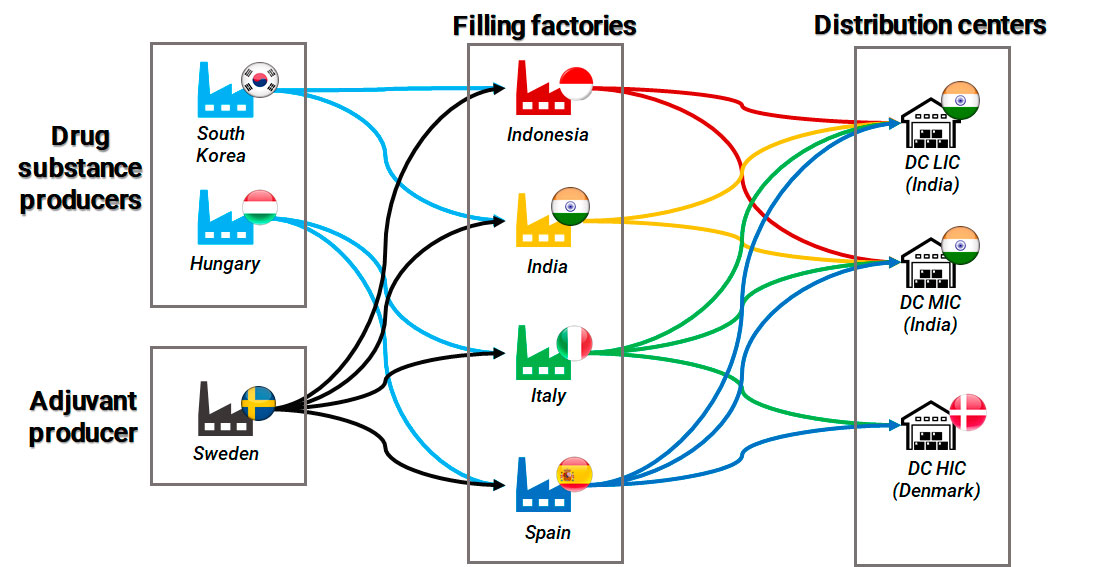
Diagram of the modeled COVID-19 supply chain
Demand
It was assumed that the total demand for the COVID-19 vaccine was equal to the entire population of the world. Therefore, demand was derived from maximum production capacity. In other words, a pure push production policy.
Production
In the model, every production stage for bulk, adjuvant, and filled vaccines requires certain campaign durations. The bulk manufacturing process takes place in bioreactors and it is this which sets the production capacity. (You can find more details about the model's parameters in the project’s PDF linked towards the end.)
Distribution
With regards to the distribution centers, the simulation modelers did not consider any capacity storage constraints. For the transportation of goods along the supply chain, they set certain transportation policies.
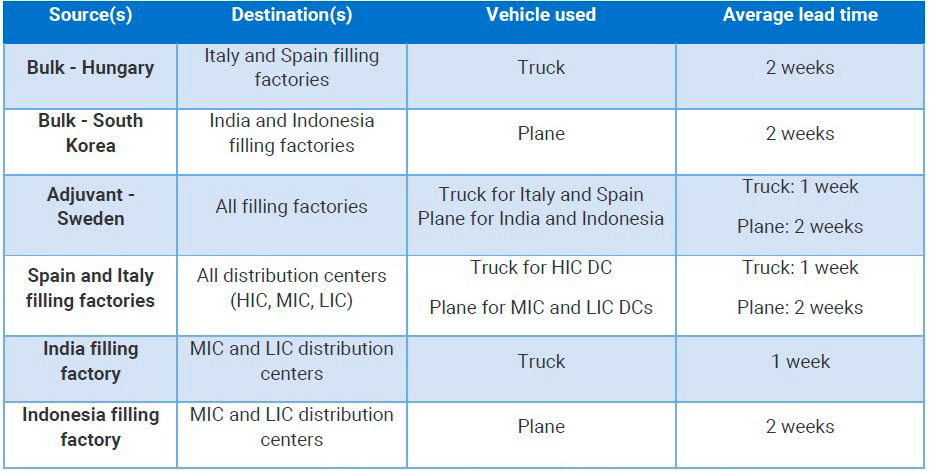
Vaccine supply chain network characteristics
After industry experts validated the model, various scenarios for the COVID-19 vaccine supply chain were run to test production configurations and logistics parameters.
The AS IS scenario contained the settings described above. Scenario A had the AS IS settings and it assumed that the third adjuvant bioreactor became active in January 2021 instead of August.
Scenario B had the previous scenario’s settings and increased bulk production (25% for the factory in South Korea and 33% for the factory in Hungary).
The consultancy team built a COVID-19 vaccine supply chain simulation in anyLogistix software with the main objective of increasing system throughput.
Simulation helped identify bottlenecks for each of the modeled scenarios. And, through further testing of the scenarios, it was possible to evaluate strategic decisions that could be implemented in the short term, given certain investments.
These decisions have a systemic impact on the other nodes of the supply chain, such as capacity utilization at the filling factories, transportation volumes, and inventories. Because of the wide range of impacts, simulation was the only holistic approach that allowed the estimation of the economic and operational impacts on the supply chain.
Supply chain modeling and simulation helped identify improvements that could accelerate the entire process of vaccine production. For example, they estimated the optimal values for order quantities and replenishment frequencies for supplier and filling factory inventory management.
Using AnyLogic software, the creation of a customized sub-model for production-based factories and then imported it into the main supply chain model in anyLogistix. This enabled consideration of detailed production parameters which can cause availability delay of the raw components at the filling factories.
The most important insight provided by the COVID-19 vaccine supply chain simulation was the final output of the system – the number of vaccine doses. To provide every person with one dose of the vaccine in a year would require six additional supply chains like the one designed in this project.
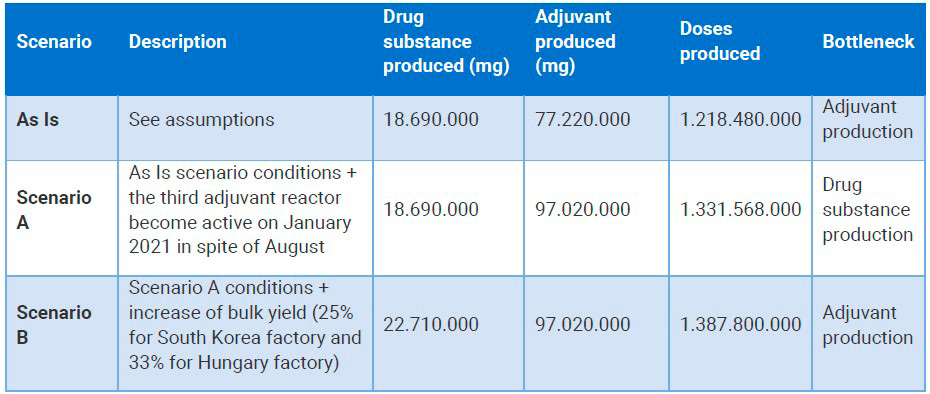
Vaccine supply chain simulation modeling results
The end-to-end supply chain visibility provided by simulation helped calculate the material flows between the supply chain nodes. It also helped define which flows should be increased or reduced to improve the final throughput of the vaccine.
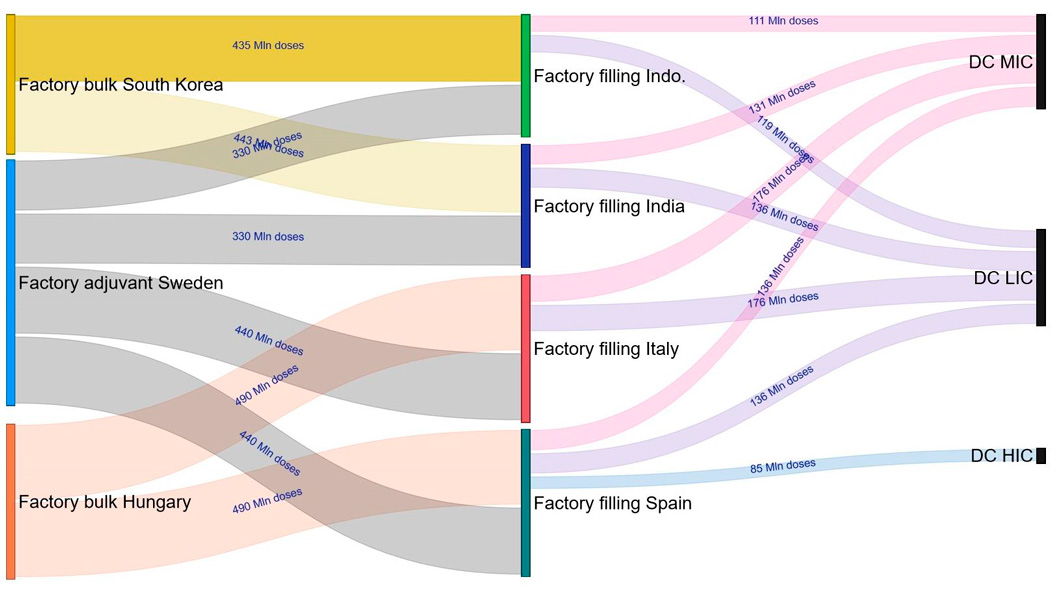
Product flows between the supply chain nodes
Finally, a great advantage of simulation is how it can help overcome time bucketing. The idea of time buckets is that they divide time, twelve months in this case, into certain periods, such as weeks or months.
Their main disadvantage is that they do not provide any visibility into what is happening inside a time bucket or what orders are in process between two periods.
Simulation modeling overcomes this time bucket limitation by considering time as a continuous dimension, as in the real world.
Simulation enables dynamic experimentation in a virtual space where you can see how a system behaves over time. You can stop the simulation at any moment and observe what is happening within any given part of all the supply chain nodes at that specific point in time.
Read the full Skkip case study by Pietro Negri and Elisa Panzeri [PDF]:
COVID-19 vaccine supply chain: addressing the complexity through simulation.
Simulation modeling and optimization with anyLogistix help improve supply chain operations. You can learn supply chain design, optimization, and simulation for free with anyLogistix Personal Learning Edition.
You can also dive deeper into the impact that next generation supply chain design and analysis methods are having on industries around the world in our simulation and optimization white paper.